Flow cytometry: History, Components, Applications

Visualizing and identifying cells by microscopy is not enough. So, a way to quantify phenotypic differences in live cell mixtures on the basis of biological markers is needed. Therefore, flow cytometry comes into the picture. In the analytical world or medical sciences, flow cytometry has a vast array of purposes. For example, when you have to quantify markers of cell death, this technique can be very useful. This is the detection of apoptotic cells, that is the count of dead cells, and also the way for the count of viable cells. However, it has many other applications like cell sorting, immunophenotyping, cell cycle analysis, cell proliferation assays, functional assays, etc. Flow cytometry is a powerful tool that has applications in immunology, molecular biology, bacteriology, virology, cancer biology, and infectious disease monitoring.
Also check out- Difference between SEM and TEM – My Biology Dictionary
Table of Contents
HISTORY
Flow cytometry has its origin on the basis of cellular impedance, a system for counting cells using the Coulter principle. The principle states that in a fluid phase, the size of a cell may be determined by the volume of electrolyte it displaces as it passes through a pair of electrodes. Due to the passage of cells through electrodes, there is an interruption in the voltage. This interruption is recorded as a measure of the required trait. The co-evolution of the flow cytometry and specific antibodies with fluorescent dyes are the reason for the success of this technology.
INTRODUCTION
Flow cytometry is a technique that allows the counting, examining, and sorting of microscopic objects suspended in a fluid based on their optical properties. The meter we use for the same purpose is called a flow cytometer. It measures the physical and chemical characteristics of a cell. Flow cytometry employs several phenomena of physics. This includes an optical-electronic detection apparatus to analyze the physical and chemical properties of microscopic particles.
Therefore, by employing flow cytometry, multiple features of each cell can be investigated simultaneously. Hence, this allows for detailed characterization of distinct populations within the heterogenous mixture of particles.
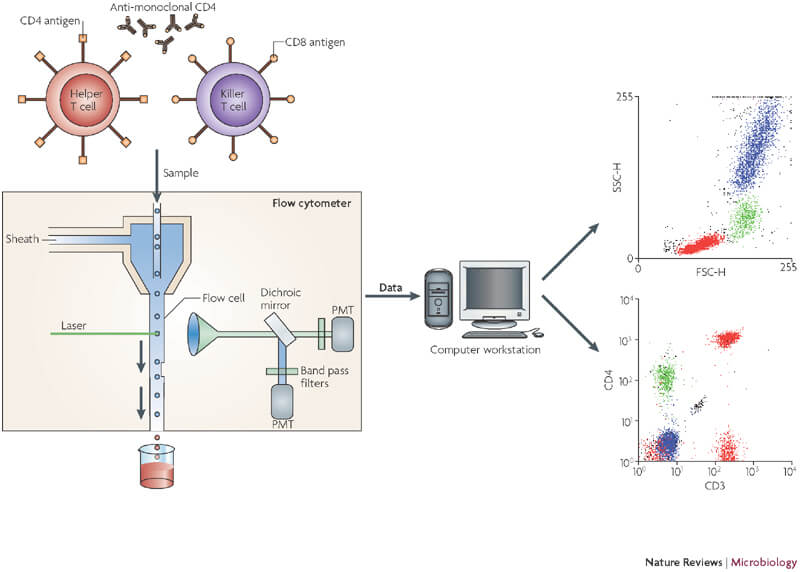
Image source: Barnett, D., Walker, B., Landay, A., & Denny, T. N. (2008). CD4 immunophenotyping in HIV infection. Nature Reviews Microbiology, 6(Suppl 11), S7-S15.
INSTRUMENTATION
Flow cytometers comprise three main components:
- Fluidics systems: this is critical to draw particles, such as blood cells, into the machine and channel them into a single file
- optical systems: this system consists of lasers and lenses that are to focus the laser beam
- Signal detector and processing systems: this converts the light signals to voltages that are then recorded
FLUIDICS SYSTEMS
The fluidics system is an integral part of a flow cytometer. Firstly, we transport the cells labelled with fluorescent antibodies to the interrogation point so that they can be excited by the lasers. The fluidics system consists of two components: a central core, through which the sample fluid is injected, and an outer sheath fluid. Secondly, these two components are pushed through the system at slightly different pressures. As the sheath fluid moves, it creates a massive drag effect on the narrowing central chamber. Therefore, altering the velocity of the central fluid. Its flow front becomes parabolic with the greatest velocity at its centre and zero at the wall. As a result, it creates a single streaming of particles or cells and is called hydrodynamic focusing.
Hydrodynamic focusing uses differential fluid flow rates to align the cell’s single file in the flow cell of a flow cytometer. In the centre stream, cells enter the flow cytometer randomly and the slower sheath fluid streams run parallel to the sample stream. This creates the laminar flow and focuses the cells in the flow cell nozzle. So, that they move through the flow cell one at a time to achieve single-cell resolution.
OPTICAL SYSTEMS
After hydrodynamic focusing, the cells move in single file in single file into the optics system. The optics are comprised of:
- The excitation optics system: consists of laser beams to scatter light and excite the fluorochrome-labelled antibodies on cells.
- The collection optics system: consists of filters, and mirrors to direct the scattered and emitted fluorescent light to a series of photomultiplier tubes(PMTs).
Photomultiplier tubes are also known as detectors or channels. These digitally convert the light into data points that are displayed in a graphical form in flow plots on the computer. In addition, some photodiodes are used which are less sensitive to light signals than PMTs. Hence, they detect the stronger Forward Scatter Channel. However, side scatter and fluorescence are weaker, thus PMTs detect these signals. To achieve the specificity of a detector for a particular fluorescent dye we place a filter in front of the tubes. So, this allows only a narrow range of wavelengths to reach the detector.
- The light scattered forward is collected by the forward scatter channel (FSC). It is used as a gauge of cell size.
- The light scattered perpendicular to the laser beam is detected as a side scatter channel (SSC). It provides information about cellular granularity and complexity.
- The plot between FSC and SSC provides valuable information about the cells.
In addition, to the information gathered by FSC and SSC channels, the optics system is responsible for filtering and directing the fluorescent light emitted by fluorochrome to the PMTs specific for those fluorochromes.
SIGNAL DETECTOR AND PROCESSING SYSTEMS
Photodetectors sense the light emitted and refracted or reflected from a single stream of particles. These photodetectors convert the light signal into a stream of electrons. Two different types of detectors are commonly in use in flow cytometers: silicon photodiodes and photomultiplier tubes. Since the photodiode is less sensitive to light signals than the PMTs, it is used to detect the stronger FSC signal. Weaker signals are detected using photomultiplier tubes that are generated by SSC and fluorescence. Moreover, histograms and dot plots represent the flow cytometry data. A histogram is used to quantify the intensity of a single parameter.
MATERIALS FOR FLOW CYTOMETRY
The materials that are used in flow cytometry are antibodies, cell activation reagents, cell purification kits, and fluorescent labels. As an example, some of the fluorescent labels can be fluorescent dyes, fluorescent proteins, toxins, etc.
APPLICATIONS OF FLOW CYTOMETRY
IMMUNOPHENOTYPING USING FLOW CYTOMETRY
Flow cytometry is useful specifically for immunophenotyping. Diagnosis of diseases, such as specific types of leukemia and lymphoma is done using immunophenotyping by flow cytometry. Therefore, this technique identifies cells on the basis of types of antigens or markers on the surface of the cell.
APOPTOSIS ANALYSIS BY FLOW CYTOMETRY
Flow cytometry is useful for rapidly quantifying markers of cell death. Measurement of the viability of cells is important for almost all flow cytometry experiments. The viability of living cells is easily assessed by DNA-binding compounds. So, these probes intercalate with DNA, but will only have access to DNA in cells with damaged membranes, in other words during cell death.
FUNCTIONAL ASSAYS
There are a number of assays that can be undertaken with flow cytometry to assess cellular functions. These can be combined with detailed cellular phenotyping to provide comprehensive datasets on cell populations present in blood and tissues.
CELL CYCLE STUDY USING FLOW CYTOMETRY
In addition to immunophenotyping flow cytometry is also used in cell cycle analysis. One of the important applications of flow cytometry is the measurement of DNA content in cells. Therefore, to measure the DNA content, the cells have to be stained with a fluorescent dye that binds to DNA in a stoichiometric manner.
Keep reading for more!
Team MBD


