Spectroscopy: Definition, Theory And Its Types

Spectroscopy is simply the study of the interaction of electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. It is the detailed study of colour as generalized from visible light to all electromagnetic spectrum bands. It allows the investigation of matter on the molecular and atomic scale. Thus allowing the composition, physical structure, and electronic structure of matter to be studied thoroughly. In this article, we will throw some light on the types of spectroscopy.
Another reading- Confocal Microscopy – My Biology Dictionary

Image source: leverageedu.com
Table of Contents
Definition Of Spectroscopy
Spectrometry is the technique used to assess the concentration or amount of a given species or matter. In those cases, the instrument that performs such measurements is called a spectrometer or spectrograph. is often used in physical and analytical chemistry. It is generally for the identification of substances through the spectrum emitted from or absorbed by them.
History Behind Spectroscopy
Historically spectroscopy referred to the use of visible light dispersed according to its wavelength, e.g. by a prism. Later the concept was expanded greatly to comprise any measurement of a quantity either a function of wavelength or frequency. This science began with Isaac Newton splitting light with a prism in his experiments with light and was called optics. Therefore, it was originally the study of visible light that later under the studies of James Clerk Maxwell came to include the entire electromagnetic spectrum.

Image source: www.pasco.com
Newton applied the word “spectrum” to describe the rainbow of colors that combine to form white light and that are revealed when it is passed through a prism. During the early 1800s, Joseph von Fraunhofer worked to enable spectroscopy to become a more precise and quantitative scientific technique. His efforts made experimental advances with dispersive spectrometers for the advancements in this field. Since then, it has played and continues to play a significant role in chemistry, physics, and astronomy.
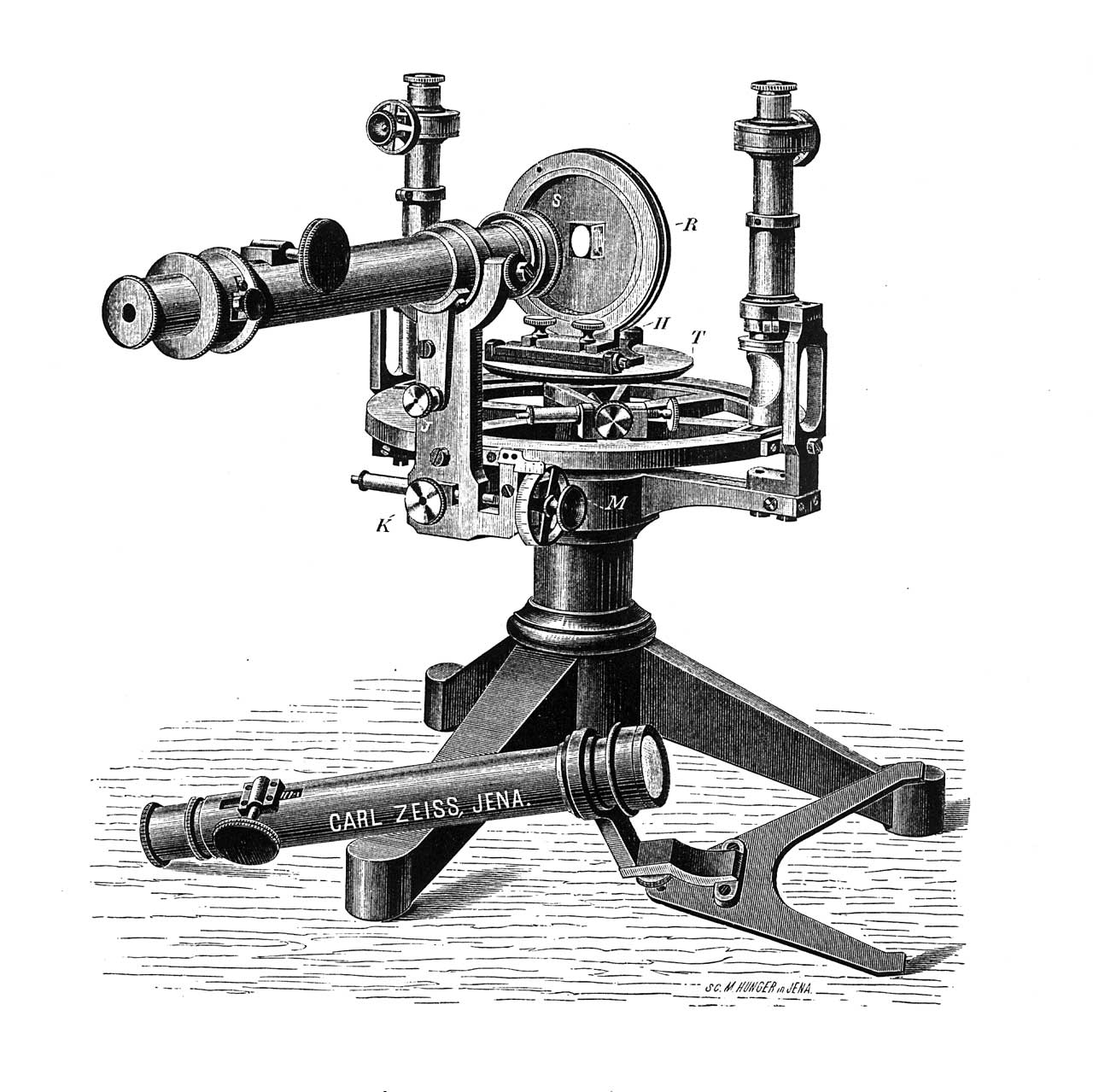
The first spectrometer based on Abbe’s calculation. Image source: www.zeiss.com
The Theory Of Spectroscopy
Quantum mechanical systems demonstrate particles such as an atom, via an oscillatory source of energy such as a photon. It states the dual nature of matter. That is actually both the particle nature and wave-like nature of particles. The coupling of the two states is strongest when the energy of the source matches the energy difference between the two states.
The energy E of a photon is related to its frequency ν by E = hν where h is Planck’s constant. So a spectrum of the system response vs. photon frequency will peak at the resonant frequency or energy. Particles such as electrons and neutrons have a comparable relationship in possessing these properties. These are the de Broglie relations, between their kinetic energy and their wavelength and frequency. Therefore can also excite resonant interactions.
Types Of Spectroscopy
There are many various types of spectroscopy but the most common ones are:
1. Infrared (IR) Spectroscopy
Infrared spectroscopy offers the possibility to measure the different types of inter-atomic bond vibrations at different frequencies. Photons in the infrared region of the spectrum have characteristic energies corresponding to those of molecular vibrations. This shows that IR spectroscopy currently remains the primary tool to study the vibrational and rotational modes of molecules. Especially in organic chemistry, the analysis of Infrared Radiation absorption spectra shows what type of bonds are present in the sample. It is also an important method for analyzing constituents like fillers, pigments, and plasticizers and also analyzing polymers.
2. Ultraviolet-Visible (UV/Vis) Spectroscopy
The ultraviolet (UV) and visible regions of the electromagnetic spectrum corresponding to the energy level transitions of electrons in atoms and molecules. All atoms absorb in the Ultraviolet (UV) region because these photons are energetic enough to excite the outer electrons of the outermost shell. If the frequency is actually high enough, photoionization takes place. Therefore UV spectroscopy can be used to probe the electronic structure of molecules in a sample, subsequently enabling the identification of the compounds present in it. UV/Vis spectroscopy is particularly useful for identifying the peptide bonds, certain amino acid side chains, and certain prosthetic groups and coenzymes.
3. Nuclear Magnetic Resonance (NMR) Spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy analyzes the magnetic properties of certain atomic nuclei to determine different electronic local environments of atoms. These include hydrogen, carbon, or other atoms in an organic compound. This kind of spectroscopy is a technique used to measure the magnetic fields that exist around atomic nuclei. NMR uses radio waves to excite atomic nuclei in a sample. When the nuclei start to resonate, it is detected by the sensitive radio receivers.
We know the resonant frequency of an atomic nucleus depends on the electronic structure of the molecule of which it is a part. Thus NMR is used to help determine the structure of the compound. It is especially a powerful tool for deducing the exact nature of monomolecular organic compounds.
4. X-Ray Spectroscopy
X-ray spectroscopy involves the interaction of X-rays with atoms in a way that they get excited. When the sufficient frequency of the X-ray interacts with a substance, the inner shell electrons in the atom are excited to outer empty orbitals, or they may be removed completely, eventually ionizing the atom. The absorption or emission frequencies or energies are characteristic of the specific atom. Two other X-ray spectroscopy techniques commonly used today are wavelength-dispersive X-ray and energy-dispersive X-ray spectroscopy. Both techniques enable elemental analysis by measuring characteristic X-rays within a narrow region of the spectrum.
Also check out-The Centrifuge – Definition, Principle, Types and Applications (mybiologydictionary.com)
5. Raman Spectroscopy
Raman spectroscopy involves the inelastic scattering of photons, known as Raman scattering. Here the apparent wavelength of a photon is changed when it interacts with the sample. Raman spectroscopy utilizes the concept of inelastic scattering of light to analyze the vibrational and rotational modes of molecules. Raman scattering uses a source of monochromatic light to illuminate the sample. The energy of the photons is shifted either up or down when the laser light interacts with molecular vibrations or other excitations in the molecular system.
There are many other types of spectroscopy also that play significant roles in the studies. These include
- Fluorescent spectroscopy
- Coherent anti-Stokes Raman spectroscopy (CARS)
- Flame Spectroscopy
All these have their own terms of advantages and real-life applications. is therefore a broad field that comprises several different sub-disciplines and a wide array of techniques, each of which uses highly specialized equipment.
Keep Reading!
Team MBD
Watch more-Introduction to spectroscopy (video) | Khan Academy

.jpg)


Very informative content!!