Structure of Proteins

Proteins are large, complex molecules and are heteropolymers containing amino acids as the monomeric units which are particularly attached to form long chains. Proteins play many critical roles in the organism’s body such as they are required for the structure, function, and regulation of the body’s tissues and organs.
Table of Contents
What is meant by the structure of proteins?

Image Source: Campbell Biology 10th edition
There are 20 different types of amino acids. Proteins are not simply in a straight line. The protein strands tend to twist, bend and fold into particular shapes. The location of each amino acid in the long string of proteins determines its three-dimensional shape and its specific function.
There are four structural levels of proteins:
Primary structure
Firstly, the amino acid sequence in a polypeptide chain forms the primary structure of a protein. The first amino acid is also termed as N-terminal amino acid and the last amino acid is termed as C terminal amino acid.
Secondary structure
Secondly, the polypeptide chain segments of proteins are coiled in very specific patterns that give rise to the protein’s shape. The secondary structure comprises the coils and folds. Moreover, the stability in the secondary structure is achieved by hydrogen bonds between the constituents of the polypeptide backbone occurring in a repetitive manner specifically.
The two main types of secondary structures are α helix and β pleated sheet.
α helix
α helix is a right-handed coil that is held together by hydrogen bonding. This H-bonding is between every fourth amino acid.
β pleated sheet
The segments (2 or more) of the polypeptide chain lie parallel to each other and are termed β strands and are connected by means of hydrogen bonds. The core of globular proteins is specifically formed by β pleated sheets.

Image Source: Campbell Biology 10th edition
Tertiary structure
Thirdly, the tertiary structure is a three-dimensional view of a protein. The tertiary structure of polypeptide results particularly from interactions between R groups (the side chains) of the amino acids. It reflects the spatial coordination of secondary structural elements. The tertiary structure is the most crucial because the proteinaceous enzymes which have the role to perform many functions in our body have a tertiary structure.
The following interactions can be seen in the tertiary structure-
1. Hydrophobic interactions.
2. Weak van der Waals forces that stabilize the protein.
3. Hydrogen bonds.
4. Ionic interactions.
To summarize, in an aqueous cellular environment, these interactions are weak, but their additive effect helps give the protein attain a unique shape. Covalent bonds called disulfide bridges may also be found.
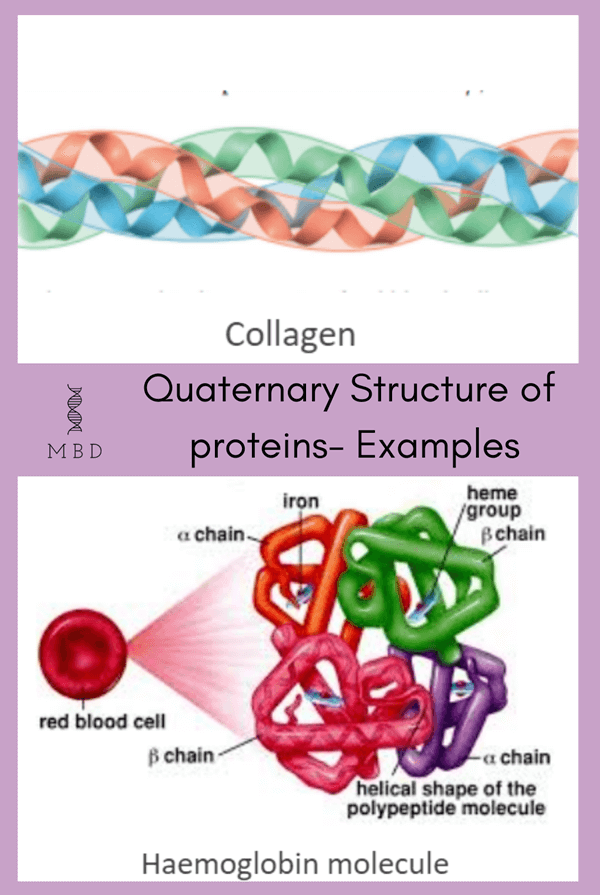
Quaternary structure
Lastly, the quaternary structure is the overall architecture of a protein that results from the aggregation of these polypeptide subunits. These are arranged in a special manner with respect to each other, for instance, spheres are arranged consecutively in the form of a plate or a cube or even spheres in a linear string. For instance, some examples of proteins with the quaternary structure are haemoglobin, collagen, etc.
There are four subunits in adult haemoglobin (Hb)-
- 2 alpha chains
- 2 beta chains
Each level of the protein structure gives rise to the next level.
Image Source: Campbell Biology 10th edition
What is the importance of the structure of proteins?
Now let’s know why these proteins form these structures. All things considered, these conformations are responsible for the catalytic and structural functions in the cell. They form enzyme complexes, protein filaments, ribosomes and membranes.
In conclusion, hope the article could explain to you the importance of four structural levels of proteins which are the machinery of living tissues.
Thank you for reading at MBD!
Team MBD
For more on Biochemistry– Biochemistry Archives – My Biology Dictionary
View 3D structure of proteins- Home – Structure – NCBI (nih.gov)